Potassium Bromide Chemical Formula Guide
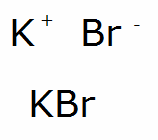
The realm of chemical compounds is vast and fascinating, with each substance having its unique properties and applications. Potassium bromide, with the chemical formula KBr, is one such compound that has been widely used in various fields, including photography, medicine, and industrial manufacturing. In this comprehensive guide, we will delve into the world of potassium bromide, exploring its chemical structure, properties, uses, and safety considerations.
Introduction to Potassium Bromide
Potassium bromide is an ionic compound composed of potassium (K) and bromine (Br) ions. The chemical formula KBr indicates that one potassium ion is bonded to one bromide ion. This compound is highly soluble in water and is often used in its crystalline form. The production of potassium bromide involves the reaction of potassium carbonate with bromine, which yields potassium bromide and carbon dioxide.
Chemical Reaction: K2CO3 + Br2 → 2KBr + CO2
Chemical Structure and Properties
The chemical structure of potassium bromide consists of a potassium ion (K+) and a bromide ion (Br-), held together by electrostatic forces. This ionic bond is responsible for the compound’s high melting and boiling points. Potassium bromide crystallizes in a face-centered cubic lattice structure, which is characteristic of many ionic compounds.
| Property | Value |
|---|---|
| Molecular Weight | 119.00 g/mol |
| Melting Point | 734 °C |
| Boiling Point | 1435 °C |
| Solubility in Water | 53.48 g/100 mL (20 °C) |

Uses of Potassium Bromide
Potassium bromide has a wide range of applications, thanks to its unique properties. Some of the most notable uses include:
- Photography: Potassium bromide was historically used in photography as a component of emulsions for photographic papers and films. Although digital photography has largely replaced traditional film, potassium bromide is still used in some niche applications.
- Medicine: Potassium bromide has been used as a sedative and an anticonvulsant in the past. However, its use has declined due to the availability of more effective and safer alternatives.
- Industrial Manufacturing: Potassium bromide is used in the production of brominated vegetable oil, which is used as an emulsifier in soft drinks. It is also used in the manufacture of pharmaceuticals, dyes, and textiles.
Safety Considerations
While potassium bromide is generally considered to be a safe compound, it can pose some health risks if not handled properly. Some of the potential hazards include:
- Toxicity: Potassium bromide can be toxic if ingested in large quantities. Symptoms of toxicity include nausea, vomiting, and abdominal pain.
- Skin and Eye Irritation: Prolonged exposure to potassium bromide can cause skin and eye irritation.
- Respiratory Problems: Inhaling potassium bromide dust can cause respiratory problems, including coughing and shortness of breath.
Handling Precautions:
- Wear protective clothing, including gloves and goggles, when handling potassium bromide.
- Avoid ingesting or inhaling the compound.
- Wash hands thoroughly after handling potassium bromide.
Conclusion
Potassium bromide is a versatile compound with a range of applications in photography, medicine, and industrial manufacturing. Its unique properties, including high solubility and ionic structure, make it a valuable substance in various fields. However, it is essential to handle potassium bromide with care, as it can pose health risks if not managed properly. By understanding the properties, uses, and safety considerations of potassium bromide, we can appreciate the importance of this compound in our daily lives.
What is the chemical formula of potassium bromide?
+The chemical formula of potassium bromide is KBr.
What are some common uses of potassium bromide?
+Potassium bromide is used in photography, medicine, and industrial manufacturing, among other applications.
Is potassium bromide toxic?
+Potassium bromide can be toxic if ingested in large quantities, but it is generally considered to be a safe compound when handled properly.
As we continue to explore the vast world of chemical compounds, it is essential to appreciate the unique properties and applications of each substance. By understanding the properties, uses, and safety considerations of potassium bromide, we can unlock its full potential and harness its benefits in various fields. Whether in photography, medicine, or industrial manufacturing, potassium bromide is a compound that deserves our attention and appreciation.